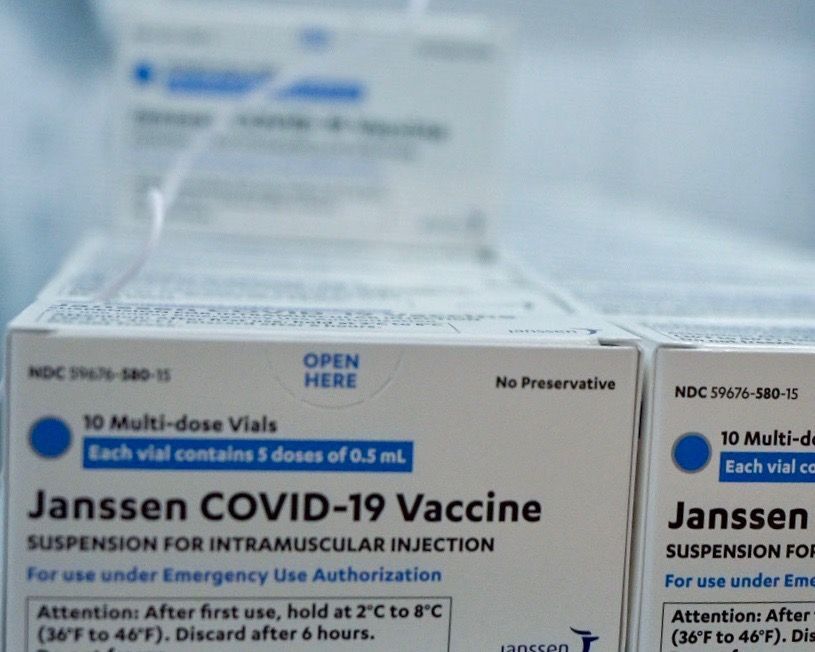
GENEVA (AN) — The World Health Organization approved Johnson & Johnson’s Janssen COVID-19 vaccine for emergency use on Friday, adding a fourth shot to the international arsenal of medicines, vaccines and diagnostics against the year-long pandemic.
The emergency use listing, which is not meant to replace the approval process of national regulators, helps expedite regulatory approval processes and enables international organizations to distribute vaccine in places where it is most needed.